(Kawasaki, Japan, 26 March 2015) Incorporating gold nanoparticles helps optimise treatment carrier size and stability to improve delivery of cancer treatment to cells, report researchers affiliated with Kawasaki INnovation Gateway at SKYFRONT, Japan.
Further information about science and technology projects at Kawasaki City is available in the Kawasaki SkyFront iNewsletter that highlights research being conducted by scientists and industries affiliated with Kawasaki INnovation Gateway at SKYFRONT (KING SKYFRONT)—the City’s flagship science and technology hub focused on open innovation in the life sciences and environment.
KING SKYFRONT is located on the opposite side of the Tama River that separates Tokyo International Airport (also known as Haneda Airport) and the Tonomachi district of Kawasaki. The Airport plays an important role in the globalization of the innovative activities of scholars, industrialists and City administrators based at KING SKYFRONT.
Gold nanoparticles size up to cancer treatment
Incorporating gold nanoparticles helps optimise treatment carrier size and stability to improve delivery of cancer treatment to cells.
Treatments that attack cancer cells through the targeted silencing of cancer genes could be developed using small interfering RNA molecules (siRNA). However delivering the siRNA into the cells intact is a challenge as it is readily degraded by enzymes in the blood and small enough to be eliminated from the blood stream by kidney filtration. Now Kazunori Kataoka at the University of Tokyo and colleagues at Tokyo Institute of Technology have designed a protective treatment delivery vehicle with optimum stability and size for delivering siRNA to cells.
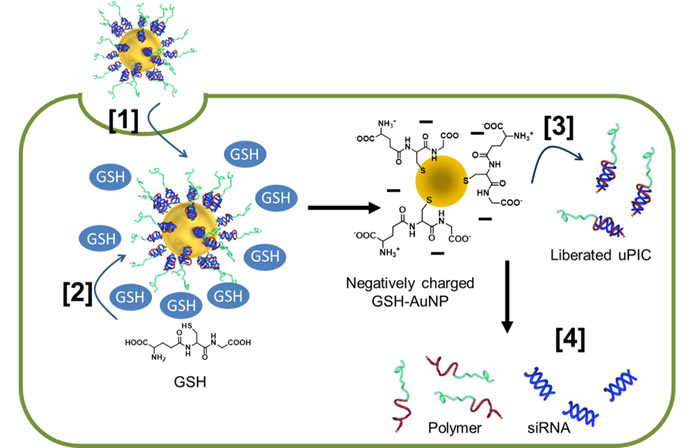
The researchers formed a polymer complex with a single siRNA molecule. The siRNA-loaded complex was then bonded to a 20 nm gold nanoparticle, which thanks to advances in synthesis techniques can be produced with a reliably low size distribution. The resulting nanoarchitecture had the optimum overall size - small enough to infiltrate cells while large enough to accumulate.
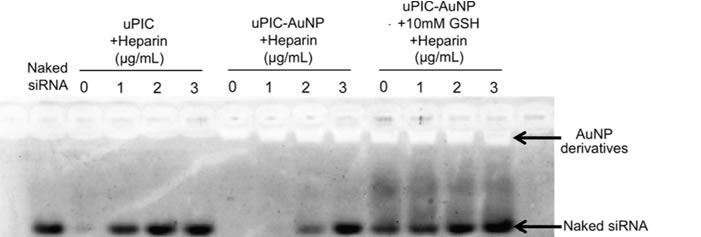
In an assay containing heparin – a biological anti-coagulant with a high negative charge density – the complex was found to release the siRNA due to electrostatic interactions. However when the gold nanoparticle was incorporated the complex remained stable. Instead, release of the siRNA from the complex with the gold nanoparticle could be triggered once inside the cell by the presence of glutathione, which is present in high concentrations in intracellular fluid. The glutathione bonded with the gold nanoparticles and the complex, detaching them from each other and leaving the siRNA prone to release.
The researchers further tested their carrier in a subcutaneous tumour model. The authors concluded that the complex bonded to the gold nanoparticle “enabled the efficient tumor accumulation of siRNA and significant in vivo gene silencing effect in the tumor, demonstrating the potential for siRNA-based cancer therapies.”
Publication and Affiliation
Hyun Jin Kim1, Hiroyasu Takemoto2, Yu Yi3, Meng Zheng4, Yoshinori Maeda3, Hiroyuki Chaya4, Kotaro Hayashi3, Peng Mi2, Frederico Pittella4, R. James Christie4, Kazuko Toh4, Yu Matsumoto4, Nobuhiro Nishiyama2, Kanjiro Miyata4* and Kazunori Kataoka1,3,4,5* Precise Engineering of siRNA Delivery Vehicles to Tumors Using Polyion Complexes and Gold Nanoparticles. ACS Nano, 9, 8979-8991, (2014).
- Department of Materials Engineering, Graduate School of Engineering, The University of Tokyo, Tokyo 113-8656, Japan
- Polymer Chemistry Division, Chemical Resources Laboratory, Tokyo Institute of Technology, Yokohama 226-8503, Japan
- Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Tokyo 113-8656, Japan
- Center for Disease Biology and Integrative Medicine, Graduate School of Medicine, The University of Tokyo, Tokyo 113-0033, Japan
- Center for NanoBio Integration, The University of Tokyo, Tokyo 113-8656, Japan