Research Highlights
Vol.6, February 2016
Precision design to kick-start the healing process
Research into developing targeted therapeutics for regenerative medicine has gained momentum in recent years. The ability to artificially trigger natural wound healing and tissue regeneration processes in the body, for example, is one of many potential applications for such therapies.
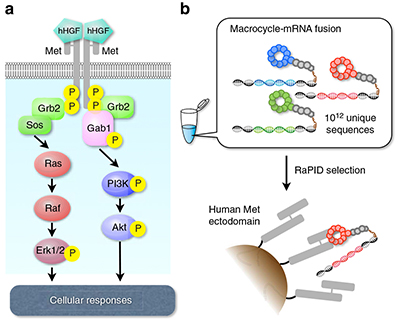
Now, Hiroaki Suga at the University of Tokyo and Kunio Matsumoto at Kanazawa University, together with scientists across Japan, have designed a peptide molecule capable of triggering a human growth factor (HGF) signalling pathway directly involved in activating tissue and wound healing.
To trigger the signalling cascade that prompts this cell activity, HGF interacts with its receptor tyrosine kinase called Met to form a ‘dimeric’ (or paired) molecular complex, which then activates the signalling cascade. The ability to pre-empt this interaction and trigger the signalling cascade artificially presents a valuable therapeutic target.
The team wanted to design a suitable peptide molecule that would bind to Met and mimic the functional role of HGF. They sifted through thousands of possible molecule designs using the Random non-standard Peptide Integrated Discovery (or RaPID) system. A key goal was to create a stable molecule that could activate the Met signalling cascade without causing unpredictable responses in patients.
They selected three dimeric macrocyclic molecules capable of binding tightly with Met and tested their effectiveness at triggering the signalling cascade in human, mouse and canine cells. In collagen gels comprising human cells and macrocycle concentrations between 10nM and 100nM, the team found cell proliferation and branching morphogenesis (the process by which a developing structure takes shape) were successfully induced. The macrocyclic molecules proved specific to human Met.
Their results represent a promising first step in creating non-protein-based regenerative medicines for repairing tissue damage and other potential applications. Also, the RaPID system shows great potential as a method for searching for other peptides that could feasibly act as triggers for other signalling cascades in future.
Reference and affiliations
- K. Ito1, K. Sakai2, Y. Suzuki2, N. Ozawa1, T. Hatta3, T. Natsume3, K. Matsumoto2 & H. Suga1.
Artificial human Met agonists based on macrocycle scaffolds. Nature Communications 6 (6373) (2015) - Department of Chemistry, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan.
- Division of Tumor Dynamics and Regulation, Cancer Research Institute, Kanazawa University, Kanazawa 920-1192, Japan.
- National Institute of Advanced Industrial Science and Technology, Biological
Information Research Center, Tokyo 135-0064, Japan.
Correspondence: kmatsu@staff.kanazawa-u.ac.jp or hsuga@chem.s.u-tokyo.ac.jp
Figure: