Research Highlights
Vol.1, August 2014
Safer cells progress spinal treatment research A stem cell treatment is developed that helps spinal injury recovery without use of ethically controversial fetal cells.
Recent research demonstrated improved spinal injury recovery in rodents and non-human primates injected with stem cells. However, these cells are derived from fetuses raising ethical issues that have stalled further progress with clinical trials involving humans. Hideyuki Okano, from Keio University, and colleagues in Japan have since developed another type of stem cell that can also provide effective treatments for spinal injuries.
The approach uses neural cells derived from clones where the ability to differentiate into a number of different cell types is induced - ‘induced pluripotent stem cells’ (iPSCs). The safety of these cells has already been evaluated. The neural stem/progenitor cells derived from iPSCs were injected into marmosets nine days after a spinal injury inflicted just below the skull. Another group of marmosets were injected with phosphate buffered saline solution nine days after injury as controls.
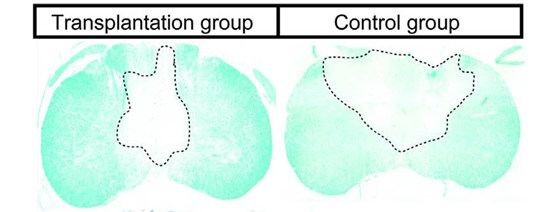
The marmosets were monitored in terms of mobility and hand grip strength. Following paralysis immediately after the injury, the animals began to recover mobility, but animals from the control group remained limited to around 60-70% of the recovery of marmosets injected with iPSCs-derived neural stem/progenitor cells. Magnetic resonance imaging (MRI) also revealed the control group had greater damage to the myelin sheath that facilitates the neuronal functions through the insulation of neuronal axons.
Twelve weeks after the injury the animals were killed and the spinal cord tissue analysed further. The injected cells were shown to differentiate into three different types of neural cell with no tumors forming, a common concern for human iPSC-based treatments.
The authors’ report on the work concludes that these human iPSCs-based cell therapy hold great promise as a cell source for the study and treatment of human diseases and disorders. However they add, “It is critical to carefully assess safety concerns prior to their use in the clinic.”
Publication and Affiliation
Yoshiomi Kobayashi1,2†, Yohei Okada1†, Go Itakura1,2, Hiroki Iwai1,2, Soraya Nishimura1,2,
Akimasa Yasuda1, Satoshi Nori1, Keigo Hikishima2,3, Tsunehiko Konomi1,2, Kanehiro Fujiyoshi4,
Osahiko Tsuji5, Yoshiaki Toyama1, Shinya Yamanaka6, Masaya Nakamura1*, Hideyuki Okano2* Pre-Evaluated Safe Human iPSC-Derived Neural Stem Cells Promote Functional Recovery after Spinal Cord Injury in Common Marmoset without Tumorigenicity. PLoS ONE, 7 e52787, (2012).
- Department of Orthopedic Surgery, School of Medicine, Keio University, Tokyo, Japan
- Department of Physiology, School of Medicine, Keio University, Tokyo, Japan
- Central Institute for Experimental Animals, Kanagawa, Japan
- Department of Orthopedic Surgery, National Hospital Organization Murayama Medical Center, Tokyo, Japan
- Department of Orthopedic Surgery, Saitama Social Insurance Hospital, Saitama, Japan
- Center for iPS Cell Research and Application (CiRA), Kyoto, Japan
* E-mail: masa@a8.keio.jp; hidokano@a2.keio.jp
† These authors contributed equally to this work.
Figure: