Research Highlights
Vol.19, December 2020
Liver blood vessel stealth coating assists the successful delivery of nanomedicines
A biologically safe organic polymer coating applied to liver scavenger blood vessels (sinusoids) to inhibit the unwanted capture of nanomedicines by the liver resulted in redirecting to specific target tissues in the human body—an important advancement in nanotechnological drug delivery
The concept of nanomedicines involves the use of nanoparticles for transporting pharmaceutical substances to intended tissues. Nanomedicines have successfully been applied to deliver a wide range of therapeutic and diagnostic agents to specific target tissues. However, nanomedicines are subject to biological processes referred to as ‘clearance mechanisms’ that act against nanoparticles, which are seen as ‘foreign particles’ by the human body.
One such mechanism is found in liver scavenger endothelial cells (LSECs), which line the wall of liver blood vessels, abundantly expressing ‘scavenger receptors’ that can capture foreign particles including nanomedicines, and remove them from the blood circulation.
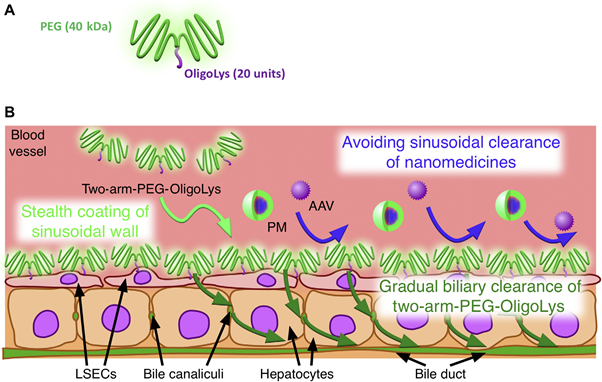
Now, Kazunori Kataoka, Director General of the Innovation Center of NanoMedicine (iCONM) administered by Kawasaki Institute of Industrial Promotion, and his team from iCONM, and collaborators at the National Institute of Quantum and Radiological Science and Technology (QST) and Department of Bioengineering, School of Engineering, The University of Tokyo, report on the successful suppression of the clearing mechanisms of LSECs by applying a stealth coating agent to liver blood vessels that inhibits the scavenger receptor activity, as a result, nanomedicines are no longer captured by the liver before being delivered to specific organs (Figure 1).
The idea of ‘stealth coating’ of nanomedicines has been widely used to suppress LSEC-mediated clearance. However, depending on the formulation and drug type, it is often difficult to obtain sufficient stealthiness of nanomedicines to completely inhibit LSEC clearance. To address this issue of LSEC-mediated capture, previous studies have attempted to saturate scavenging functions of LSECs by preinjecting empty nanoparticles and scavenger receptor inhibitors such as polyinosinic acid. However, receptor saturation strategies often induce inflammatory responses, hence they are not clinically viable. Furthermore, scavenger receptor inhibitors block only the specific receptor that they target even though LSECs have different types of scavenger receptors, which often recognize the same nanomedicine, implying that various clearing mechanisms need to be inhibited simultaneously. To overcome these issues, Kataoka and his team developed an approach to block the diverse clearance pathways of liver sinusoidal scavenger walls by coating them with polyethylene glycol (PEG), the first -of-its-kind strategy in the world.
The PEG coating must satisfy two primary requirements. First, the coating must be selective to the liver sinusoidal wall because coating to extra-liver blood vessels not only causes adverse toxicity but also decreases the amount of nanomedicines delivered to target organs. Second, the coating must be temporary as long-term coating affects normal liver functions. To meet these prerequisites, the team precisely designed a coating agent with two-armed PEG conjugated to positively charged oligolysine (two-arm PEG−OligoLys) (Figure 1A), which demonstrated selective coating to the liver sinusoidal wall, leaving other tissue endothelium uncoated, thus accessible to the delivery of nanomedicines. Interestingly, the two-arm PEG−OligoLys coating is gradually removed from the wall and is excreted to the bile within six hours, while its counter variant one-armed PEG−OligoLys do not de-coat the wall. Therefore, the precise molecular design of the two-arm PEG−OligoLys satisfied both selective and transient stealth coating.
The selective and transient liver scavenger wall stealth coating by the two-arm PEG−OligoLys efficiently prevents the sinusoidal clearance of both viral and nonviral gene vectors, representatives of nature-derived and artificially-engineered nanomedicines, respectively, thereby boosting their gene delivery efficiency in the target tissues (Figure 1B). Despite the clinical success of viral vectors, liver sinusoidal capture drastically hinders the ability of viruses to reach their target organs, thus resulting in the use of high viral dose—often leading to death humans—to obtain a therapeutic level of protein expression.
Two-arm PEG−OligoLys coating successfully suppressed the liver sinusoidal capture of adeno-associated virus serotype 8 (AAV8), and as a result, dramatically improved the gene transfer efficiency to their intrinsic target organs, heart, and skeletal muscle. Also, the researchers extended their strategy to a plasmid DNA encapsulated smart nanomachine (polyplex micelle; PM), a model nonviral gene delivery system, which allows economically feasible and safe gene therapy. Two-arm PEG−OligoLys precoating suppressed the adsorption of DNA nanomachine to the liver sinusoidal wall thus resulting in a 10-fold improvement in gene delivery efficiency to the cancer site.
Quoting the scientists: “Our approach is versatile for combinational use with various nanomedicines, including synthetic and nature-derived nanomedicines, opening avenues for future nanotherapy and nanodiagnosis.”
Reference
Dirisala, A. et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines
Sci. Adv. 2020; 6 : eabb8133
https://advances.sciencemag.org/content/6/26/eabb8133
DOI: 10.1126/sciadv.abb8133
Figure: [Fig. 1 from the original article]