Research Highlights
Vol.11 January 2018
High precision drug delivery across blood-brain barrier using nanocarriers with surfaces functionalized with glucose
Medical approaches to accurately deliver pharmaceutical drugs to specific targets, such as cancerous tissue, would benefit patients, for example by reducing side effects. Recently, surface functionalization of so-called ‘nanocarriers’ has been developed to be an innovative way for delivering drugs for therapeutic effects in patients, especially to target sites.
However, target sites with blood vessels that have low permeability, such as the blood–brain barrier (BBB), pose an obstacle to delivering nanocarriers and their loads. Attempts at designing nanocarriers that can cross the BBB have resulted in very poor delivery (1% dose/g-brain) or unreported delivery levels. Researchers in Japan have recently designed nanocarriers that can cross the BBB into the brain with enhanced and controllable efficiency.
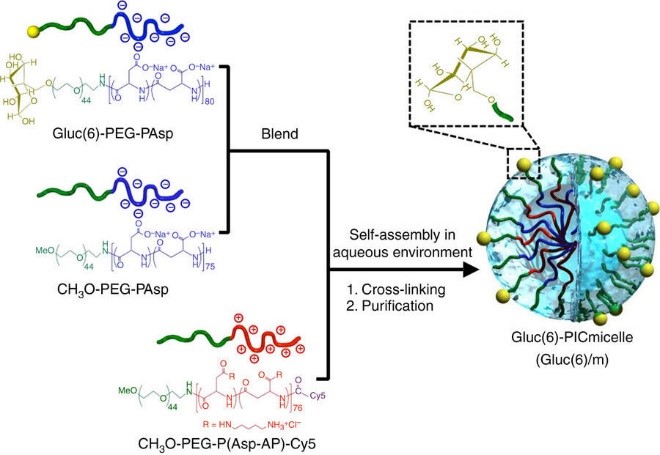
Kazunori Kataoka, Takanori Yokota and colleagues at the Kawasaki Institute of Industrial Promotion, Tokyo Medical and Dental University, The University of Tokyo and Tokyo Institute of Technology, developed nanocarriers for targeting glucose transporter-1 (GLUT1), which is highly expressed on the surfaces of brain capillary endothelial cells, the main constituents of the BBB. The researchers exploited the observed glycemic increase after fasting in order to enhance the uptake of the nanocarriers. Polymeric micelle nanocarriers were ‘decorated’ with glucose molecules in an orientation and density that would enhance their binding to GLUT1. Binding and uptake were demonstrated in vitro using GLUT1-overexpressing cells.
In vivo uptake was studied by injecting mice with fluorescence labelled nanocarriers. The mice were subjected to a one-day fasting state followed by injection of glucose, nanocarrier uptake by the brain increased 56-fold and reached levels of up to 6% dose/g-brain. This effect was only observed in the brain, reflecting the specificity of the strategy. Nanocarriers were detected in the brain parenchyma within 30 min of administering the glucose solution, indicating that they had crossed the BBB. Furthermore, controlling glucose density on the nanocarriers resulted in differential distribution within the brain, offering the potential for specific targeting.
The authors conclude that “the clinical relevance of the present results should be further examined by using animal models that properly reflect the BBB structure in each disease and by optimizing the structure of the glucosylated nanocarriers and the condition of glycemic control.”
Publication and Affiliations
Y. Anraku1, H. Kuwahara2,3, Y. Fukusato1, A. Mizoguchi4, T. Ishii1, K. Nitta2,3, Y. Matsumoto 4,5, K. Toh6, K. Miyata4,7, S. Uchida1,4, K. Nishina2,3, K. Osada1, K. Itaka4, N. Nishiyama8, H. Mizusawa2,3, T. Yamasoba5, T. Yokota*2,3, K. Kataoka*6,9 . Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nature Communications 8 (2017) DOI: 10.1038/s41467-017-00952-3
- Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan.
- Department of Neurology and Neurological Science, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan.
- Center for Brain Integration Research, Tokyo Medical and Dental University (TMDU), 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan.
- Center for Disease Biology and Integrative Medicine, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
- Department of Otorhinolaryngology and Head and Neck Surgery, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
- Innovation Center of NanoMedicine, Kawasaki Institute of Industrial Promotion, 3-25-14 Tonomachi, Kawasaki-ku, Kawasaki 210-0821, Japan.
- 7 Department of Materials Engineering, Graduate School of Engineering, The University of Tokyo, 7- 3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan.
- Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, R1-11, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan.
- Policy Alternatives Research Institute, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. Y. Anraku, H. Kuwahara and Y. Fukusato contributed equally to this work.
Corresponding authors, T.Y. (email: tak-yokota.nuro@tmd.ac.jp) or K.K. (email: kataoka@bmw.t.u-tokyo.ac.jp)
Figure: