Research Highlights
Vol.19, December 2020
A new potential approach for treating post-infarct heart failure
A particular type of human cardiac fibroblast is found to improve the heart function in post-infarct heart failure rats
Cardiovascular diseases — involving the heart or blood vessels — are a major global cause of death. Specifically, injuries causing restricted blood supply to organ tissue lead to drastically degraded organ function, which can be followed by heart failure. There is plenty of evidence that cardiac fibroblasts (CFs) are particularly critical for maintaining heart function in times of both health and disease. But detailed understanding is lacking, partly because CFs have various origins, and so exist as a heterogeneous population. Now, Takahiro Iwamiya from Metcela Inc. and colleagues have analyzed the CF population, and have identified a subpopulation that improves heart systolic function by triggering lymphangiogenesis.
The researchers hypothesized that one subpopulation of CFs might have the capability to repair the myocardium — an idea inspired by the notion that CFs contribute both ‘positively’ and ‘negatively’ to heart function in cardiac development, repair, and pathogenesis. The former is for example evidenced from CFs’ essential role in the structural organization of the tissue by regulating extracellular matrix (ECM) homeostasis and modulating the proliferation/cell death balance, autophagy, adhesion, and migration of numerous other types of cells. The latter for example from CFs’ leading cause of cardiac fibrosis in pathological states. In other words, CFs show different characteristics for different conditions of the heart tissue environment.
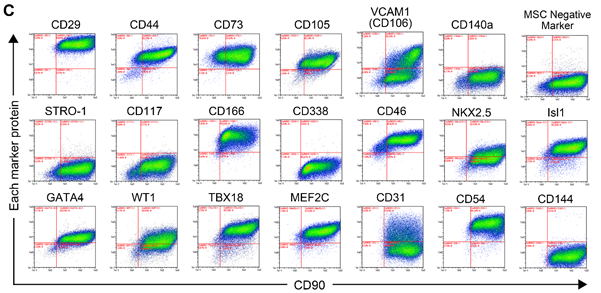
Iwamiya and colleagues worked with human fetal CFs. They investigated the characteristics of CFs by high-throughput cell profiling for the co-expression of CD90 (fibroblast marker) and various heart lineage cell markers to reveal their heterogeneity. Almost all markers examined were expressed in a unimodal way, except for one marker called VCAM1 (‘vascular cell adhesion molecule 1’), which was expressed with a bimodal distribution, meaning that the CFs consisted of at least two distinct populations with different properties (Fig 1).
The scientists then studied whether the CFs expressing or not VCAM1 had different effects on cardiac function. By bioinformatics analysis, they first found that VCAM1-positive CFs (VCFs) expressed numerous genes related to angiogenesis (the formation of new blood vessels from existing vessels) and lymphangiogenesis (the similar formation of new lymph vessels, lymph being the liquid circulating through tissue). Conversely, many of the genes expressed at higher levels in VCAM1-negative CFs (VNCFs) were linked to fibrotic tissue formation. Then, they observed the VCF’s ability of endothelial or lymphatic endothelial vascular network formation. Lymphatic vascular formation assays showed significant stimulation of cardiac lymphatic cell network complexity, induced by VCFs compared to VNCFs. Recent studies have suggested that lymphangiogenesis improves cardiac function and repair by draining accumulated fluids and maintaining the physiological interstitial fluid equilibrium. They hypothesized that VCFs may restore cardiac function in heart failure following myocardial infarction by enhancing lymphatic vessel formation.
Comparing the VCF-treated with the placebo-treated rats (vehicle control), Iwamiya and colleagues noted that the heart systolic function and mechanical properties of ventricular walls were substantially increased in the former. By examining the hearts after 18 weeks of treatment, the scientists concluded that very likely, VCFs can restore cardiac function after heart failure particularly because they recruit lymphatic endothelial cells to the infarcts.
The current findings are highly encouraging. Citing Iwamiya and colleagues: “These results demonstrate that a specific population of cultured human fetal heart-derived fibroblasts expressing VCAM1 has a role in lymphangiogenesis, and that a treatment with VCFs restores cardiac contractile functions on heart failure following myocardial infarction …” and “ … understanding the molecular mechanism mediating lymphangiogenesis may provide new insights in cardiac development and pathogenesis, and lead to new options in the field of heart regeneration.”
Reference
Takahiro Iwamiya, Bertrand-David Segard, Yuimi Matsuoka, and Tomomi Imamura, Human cardiac fibroblasts expressing VCAM1 improve heart function in postinfarct heart failure rat models by stimulating lymphangiogenesis.
PLoS ONE 15(9): e0237810 (2020)
https://doi.org/10.1371/journal.pone.0237810
Figure: 1