Research Highlights
Vol.18, March 2020
Packaging single-stranded DNA for potential use in gene therapy
Encapsulating large size of genetic material in the form of single-stranded DNA in spherical nanoparticles expands the potential of gene therapy.
In one particular type of gene therapy, genes need to be introduced in the nucleus of target cells such that they start the production of proteins that ultimately lead to apoptosis, the process by which a cell kills itself. Getting such genes into target cells is not straightforward, however: barriers often exist that prevent a biomolecular ‘package’ above a certain size from reaching target cells in conventional ways. Now, Kazunori Kataoka from the Kawasaki Institute of Industrial Promotion and colleagues report a promising method for packing genetic material in a compact way, useful for the transport of genes for gene therapy in cases of diseases where size-restriction barriers cause delivery problems.
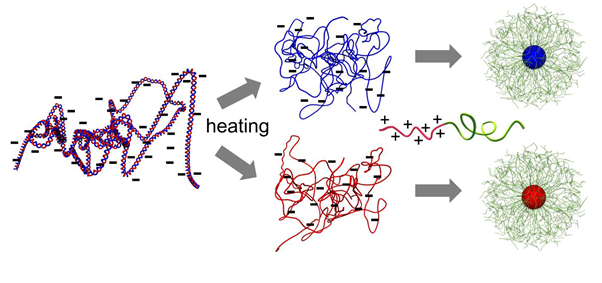
The scientists were inspired by the adeno-associated virus (AAV), a small virus that has its DNA packed in single-stranded form (ssDNA) — most of the time, DNA presents itself as a double-stranded helix. Importantly, the ssDNA in the AAV is transcriptionally active, meaning that it can be copied into RNA, which can then lead to the production of proteins.
Kataoka and colleagues first heated double-stranded DNA, resulting in the unpairing of the two strands. Single strand was then made to bind to polymers, with the structure having a spherical shape. The resulting particles are of a type known as polyplex micelles, and the researchers call them ‘MARU’ (micellar assembly retaining unparied ssDNA). By means of electron microscopy, the size of the core of the spherical MARU particles was measured to be approximately 29 nm (nanometer), making them more compact than similar, rod-like shaped micelles made from double-stranded DNA. Compared to the AAV, the MARU particles can encapsulate DNA of larger size, and have the potential to deliver a larger varieties of DNA material encoding proteins.
After confirming that MARU particles can indeed activate gene expression, the researchers tested their use as gene carriers to pancreatic tumor cells, for which the size limit for gene therapy lies around 100 nm. The particles were injected intravenously in a mouse model of pancreatic cancer. After six hours, the tumor was removed and investigated. MARU particles were detected inside the tumor, showing their delivery capability. Finally, Kataoka and colleagues performed gene therapy experiments on tumor-bearing mice. The scientists prepared MARU particles with DNA encoding cytosine deaminase, a protein that converts a compound known as 5-FC into 5-FU. The latter compound, 5-FU, stimulates apoptosis and as such is a well-known anticancer drug. The mice were also injected with 5-FC. Introducing the MARU particles led to a strong suppression effect on tumor growth, likely resulting from direct gene expression — the production of cytosine deaminase resulting in the conversion of 5-FC into 5-FU — in the tumor cells.
Apart from demonstrating the potential of gene therapy based on packing large size of ssDNA, the work of Kataoka and colleagues has fundamental appeal with respect to better understanding the gene expression power of ssDNA. Quoting the scientists: “[MARU] may have potential utility to examine underlying mechanisms involved in this fundamental function of ssDNA, which has yet to be elucidated.”
Reference:
Tockary, Theofilus A. et al. Single-Stranded DNA-Packaged Polyplex Micelle as Adeno-Associated-Virus-Inspired Compact Vector to Systemically Target Stroma-Rich Pancreatic Cancer.
ACS Nano 13,12732-12742 (2019)
https://doi.org/10.1021/acsnano.9b04676
Figure: [Scheme 1 from the paper]